The macroevolutionary consequences of island hopping
Natural historians have known for a long time that islands often harbour an extraordinary amount of biodiversity. One reason is that lineages that colonize islands can exploit open ecological niches and therefore diversify along new evolutionary trajectories. So did Anolis lizards following their arrival to the Caribbean. A range of habitat specialists has evolved repeatedly on different islands, creating a structured diversity of morphologies. Since one Anolis clade has remained on the mainland, and one clade has returned the mainland, this group of lizards presents a kind of before/after ‘island evolution’ experimental setting that can help us understand how evolution works.
Our new study, published in Nature Communications, took advantage of this setting to find out if morphological diversification proceeded in a different way following colonization of the Caribbean islands compared to the lizards that stayed on the mainland. This would be predicted if there were lots of ecological opportunities on islands. Furthermore, we wanted to know if the clade that returned to the mainland evolved along similar trajectories as their distant mainland relatives, or if they followed more closely their Caribbean relatives. Finally, we also wanted to test if evolution on the Caribbean islands was faster, more punctuated, and led to higher morphological diversity than on the mainland, again expected if lizards encountered a lot of empty niches on islands.
To answer these questions, Nathalie and Illiam travelled to the Nanoscale Research Facility at the University of Florida to collect CT-scans of more than 700 individuals of 271 Anolis species. Back home they quantified the morphology of the parts of the skeleton that determine how the lizards navigate their habitat – limb bones, hip and shoulder girdles.
Interestingly, we found that the evolutionary modularity – the covariation of parts of the locomotor skeleton across the evolutionary tree – was different between the clades: while the lizards whose ancestors had never set foot on an island showed a tight covariation between the fore- and hindlimbs, Anolis evolution on islands followed a trajectory with much tighter covariation between limbs and their respective girdles (forming a front and a hind module). What is more, the evolutionary modularity that was seen across island lizards was also seen in the mainland re-colonizers.
What does this mean? The answers are hidden in the past, of course, but we speculate that the exploitation of ecological opportunities following colonization of the islands led to a profound change in how limbs and girdles develop and grow together during ontogeny. That is, adaptation on islands may have changed how limbs and girdles vary together, and this change persisted even after lizards recolonized the mainland. This meant that lizards in the clade that recolonized the mainland produced very similar morphologies to those seen on the islands, but failed to explore some parts of the morphospace occupied by their other mainland relatives.
If we compare the island lizards with the clade that returned to the mainland, we find that a general prediction from island biogeography holds true – island lizards show faster and more variable evolutionary rates and greater morphological variation. That is, while the patterns of diversification of the locomotor skeleton is similar, the mainland lizards produce only a subset of the morphologies seen on the islands. The same is not true, however, if we compare the morphology of island lizards to the morphology of lizards in the ‘primary’ mainland clade. This mainland clade is equally diverse as the lizards on islands, and it contains morphologies that are not seen on the islands. Perhaps this means that comparing diversification patterns of island and mainland groups is only ‘fair’ if the two groups share the same variability. Or perhaps it reflects historical differences in the ecological opportunities for adaptive diversification in the two mainland clades.
We do not yet have all the answers, and many will forever remain unknown. However, we do suggest that these macroevolutionary trends of the locomotor skeleton in Anolis illustrate that adaptation to ecological opportunities on islands can have profound effects on trait development, with lasting effects on patterns of morphological diversification. To put this hypothesis to the test, we need to study how the parts of the locomotor skeleton vary together within species, and do this for several species across the different clades. A big task, but not impossible.
New paper throws doubt on a popular idea
The rock-paper-scissors story of the side-blotched lizards was beautiful. Many lizards have similar colour polymorphism, and quite a few PhD projects and postdoc years have been spent testing the idea in other species. One of the most obvious candidates are wall lizards, where several species come with clearly distinct white, yellow and orange/red throats and bellies. The early reports were – as they often are – encouraging. But researchers throughout Europe grew increasingly worried that the phenotypic differences between red, yellow and white animals at best were small and inconsistent or, at worst, entirely absent.
Alternative mating strategies tend to be manifested behaviourally. We therefore decided to release common wall lizards of the three main colour morphs in large outdoor enclosures in Moulis and observe what they actually do. The prediction is simple: if the morphs signal relevant aspects of sociosexual behaviour, such differences should be evident in how the lizards interact with one another, or who they chose to mate with.
We had done similar studies before, both in Moulis and in Oxford, and it works like a dream. As long as there are people truly dedicated to watching lizards, that is. Thankfully, the lead author of the study – Javier Abalos – is very patient and, quite frankly, obsessed with lizard watching.
With a huge amount of behavioural data collected, home ranges quantified, and paternity secured, Javier could conclusively say that there is nothing going on. Nothing, Nada, Inte ett dugg.
There is of course always the possibility that lizards do something else when they go about their business outside of field enclosures. So Javier and another obsessive lizard watcher – Guillem Perez I de Lanuza – added a long term field data set to the mix. Again, no evidence that males or females of different colour morphs behave differently, or that they modify their behaviour depending on the morph of other individuals.
Conclusion: Whatever these colours mean, they certainly do not signal differences in social or sexual behaviour. At least not in this lineage of wall lizards. You can read the full story here.
The problem, of course, is that it is very difficult to prove that there is nothing going on. It is also very unpopular when data fail to support a good old adaptive story. So reviewers try hard to come up with reasons for rejection – it can almost be endearing to read attempts to rescue a favourite hypothesis. But efforts like those from Allen Moore and the editors of Ecology & Evolution ensure a swift publication of unpopular findings. Chapeau to the editor for their frank evaluation of the reviewer comments from a different journal. Such encouragement is especially important for early career researchers so they won’t be put off by people who care more about their ego than about what the results say.
Nevertheless, at the end of the day, there must be some kind of explanation for why these morphs persist. They are even found in several other wall lizard species. But after Javier’s results you would have to be an optimist beyond belief to think they signal alternative behavioural strategies. If you have different ideas and want to test them, please don’t hesitate to get in touch.
A major blow to the ’plasticity-first’ hypothesis?
 Skeletal morphology is a classic candidate for ‘plasticity-led’ evolution. Our new paper in eLife tests the idea that such ‘plasticity-led’ evolution has occurred in the adaptive radiation of Anolis lizards on the Greater Antilles.
Skeletal morphology is a classic candidate for ‘plasticity-led’ evolution. Our new paper in eLife tests the idea that such ‘plasticity-led’ evolution has occurred in the adaptive radiation of Anolis lizards on the Greater Antilles.
In her study, Nathalie quantified the morphological divergence between ecomorphs on the four Greater Antillean islands. She then compared this to the morphological changes induced by microhabitats that promote climbing or running. It turns out that divergences between ecomorphs have occurred in parallel on the different islands. However, the divergence between ecomorphs was not aligned with plastic responses. In addition, plasticity differed between species, making it too transient to exercise a persistent effect on adaptive diversification of the locomotor skeleton.
At first sight, this may appear to be a major blow to the plasticity-first hypothesis. And in some ways it is. However, rather than showing that morphological plasticity has been rather inconsequential in this adaptive radiation, Nathalie’s discussion of her results helps us sharpen our theories about the relationship between plasticity and evolution – something that we and others have argued is necessary. Step by step, this brings us one step closer to actually understanding how development shapes evolution.
Does evolution go where plasticity leads?
Mary-Jane West-Eberhard famously suggested that plasticity ‘takes the lead’ in adaptive evolution. But how can you tell if you are not there to see it happen? In a new paper in Evolution Letters, we show one way to tackle the problem – here is a quick summary of what we did and what we found.
We figured that we can look for signatures of plasticity by comparing the magnitude and direction of plastic responses to the environment with how locally adapted populations differ.
The literature on plant local adaptation is full of reciprocal transplant experiments that record both plasticity and adaptive divergence. We therefore used such data to test a number of hypotheses – and here is what we found:
First, the ancestral plastic response was generally well aligned with the difference between locally adapted phenotypes, making plasticity appear to ‘take the lead’ in adaptive evolution. Or – if you prefer – the plastic response of ancestors left a signature in the locally adapted phenotypes of descendants.
Second, plastic responses were sometimes more extreme than the locally adapted phenotypes. This can give the false impression that plasticity and evolutionary adaptation oppose each other: in fact, truly maladaptive plasticity was rare.
Third, although the signature of plasticity generally persists during local adaptation, genetic evolution modified trait combinations independently of plasticity.
We learnt a lot during this study. Perhaps the most important message is that we are a long way from understanding the relationship between plasticity and evolution – largely because very few studies are actually designed to test if evolution goes where plasticity leads. Hopefully this paper shows not just that this is a hypothesis that can be tested, but also help to clarify what kind of data that we need.
The first case of vertical transmission of a nematode from lizard mums to their offspring
The common perception of what scientist generally do probably includes making discoveries. But, in fact, the real life of a scientist mostly consists of collecting data, analyzing and interpreting data, reading and writing articles. Rarely are these activities interrupted by making discoveries, but it does happen.
While at Oxford, we began a project that investigated how lizard embryos in non-native populations in England cope with the cool climate. Unlike birds, who care for their offspring, most lizards bury their eggs in the soil. To study the adaptations that allow wall lizard embryos to develop at low temperatures, Nathalie spent most of the spring 2015 dissecting lizard eggs. Under the microscope, she opened the leathery egg shell with fine dissection tools and carefully detached the tiny embryo from the yolk. And she did this for hundreds of eggs.
The routine was disturbed one day by an odd discovery: in one of the embryos, Nathalie saw that something worm-like was wriggling in the brain of a baby-lizard! The discovery raised lots of excitement among her colleagues, but it also raised lots of questions: Why is the worm there and what is it actually? How did it get there – was it an accident or is this how the worm lives? Can the baby-lizard survive with worms wriggling in its brain?
Since we are lizard biologists rather than worm biologists, we teamed up with people who know more about nematodes with the aim to answer at least some of these questions. The result of this research can be read in our recent publication in the American Naturalist and we also wrote a little blog post that explains what answers we found. It is super cool and hopefully someone wants to pick this up and continue from where we left it!
Transposable elements unleashed
Mobile DNA sequences – transposable elements or TEs for short – are found in the genome of virtually all organisms. As their name implies, TEs can cut or copy themselves from one location in the genome to another. This can wreak havoc as insertion of TEs may interfere with gene regulation or in fact knock out entire genes. Cells therefore have mechanisms that prevent TEs from jumping, including DNA methylation and other epigenetic tools. Thus, TEs are not roaming freely through the genome, but are restricted from entering functionally important parts. Preventing TE invasion is particularly important when genes are regulated through spatial proximity to each other. The textbook example of this situation are the Hox genes, which are the key players in embryonic development with an ingenious mode of action: Hox genes are arranged in tight clusters and their position in the cluster defines their time and space of expression, and thus their effect on the patterning of the early embryo. It is therefore fitting that Hox gene clusters of mammals and other well-studied vertebrates have been found to be almost completely free of TEs. Nathalie’s new study reveals that Anolis lizards have broken this paradigm. Moreover, the invasion of TEs into Hox clusters of Anolis lizards can be linked to aberrant gene expression and increased rates of speciation.
Ever since the discovery of TEs, people have speculated about their evolutionary implications. One possible consequence of high TE activity is structural genomic variation. This may accelerate genomic incompatibility between populations; effectively making TEs engines of speciation.
Occasionally, TE insertions may also generate phenotypic novelty. As noted above, some genes are regulated through its proximity to other genes, which means that invasion of TEs can change expression of a number of genes simultaneously. Furthermore, since jumping TEs often drag along neighbouring genomic regions, they can translocate regulatory sequences that cause genes to be expressed in new cell types or at different stages in development.
While these are good reasons to expect TEs to promote evolution, examples are few and their role often appears idiosyncratic. An excellent group for a more systematic survey of TE-driven diversification are squamate reptiles, a group that includes lizards and snakes. Squamate genomes do not only appear particularly rich and variable in TEs, but their body plan is also highly malleable. Illustrative examples include the adaptive radiation of Anolis lizards and the repeated evolution of limbless and elongated bodies.
Nathalie decided to study how TEs have shaped the genomes, and in particular, the Hox clusters, of squamates. Her first surprise was to discover that lizards possess more Hox genes than all other tetrapods since they retained some genes that other lineages have ditched. The second surprise came when she looked at the TE content of Hox clusters. Despite the high TE content in their genomes, squamates follow other vertebrates in generally protecting their Hox clusters from TEs. But there was one exception: Nathalie found massive invasion of TEs in the Hox clusters of two out of three Anolis species, with TE contents almost as high as the average place in the genome.
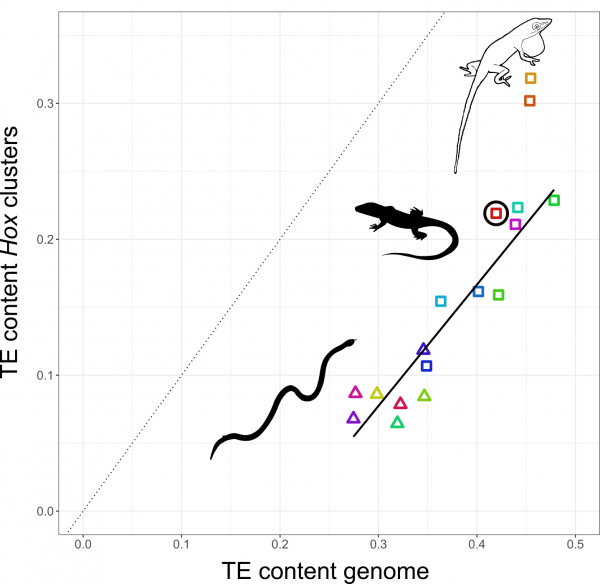
The relationship between TE content in Hox clusters relative to genome-wide TE content in squamate reptiles. All lizards and snakes restrict TEs from their Hox clusters down to roughly half the genome-wide average. However, two Anolis species show Hox clusters that are invaded by TEs, while a third Anolis species (black circle) follows the general trend.
Why in Anolis? Anolis lizards are famous in evolutionary biology due to their adaptive morphological radiation involving high rates of speciation – amassing close to 400 species. In a previous study Nathalie showed that Anolis lineages with more speciation events in the past have more TEs in their Hox clusters. This new genome-wide study reveals that this signature of speciation is indeed pronounced in Hox clusters: only the two Anolis species from amply speciating lineages exhibit unusually TE-rich Hox clusters, while a third species (Anolis auratus, black circle in figure above) follows the norm and keeps its Hox clusters relatively free from TEs. Looking in detail at genome-wide TE landscapes of these three Anolis species, Nathalie discovered that the two species with TE-rich Hox clusters had a larger population of young, more active TEs in their genomes. In addition, the inferred timing of peak activity of these TEs broadly coincided with past speciation events.
These results suggest that – during speciation events – TEs are unusually active and proliferate throughout the genome. As a result, even crucial regions such as Hox clusters become invaded. Subsequently, TEs are removed from Hox clusters by selection until a ‘healthy equilibrium’ of TE content relative to the genome-wide TE content is reached. This equilibrium appears highly conserved as the Hox clusters of almost all lizards and snakes contain close to 50% of the global TE content. This proposed model generates a number of predictions that can be tested with genomic data from lineages with variable rates of speciation.
How then do some Anolis species cope with having their Hox clusters invaded by TEs? Clearly, the inflation of Hox clusters – increasing the distance between genes – does not disrupt the patterning of the early embryo. Genes located at one end of the cluster remain expressed early in the head of the embryo, while genes located at the other end are expressed late in the tail. However, the successive activation of Hox genes predicts that disruption, if occurring at all, should be most pronounced towards the end of the Hox clusters. Nathalie found that this indeed is the case: one out of four Hox13 genes showed aberrant expression in the two Anolis species with TE invaded Hox clusters, but this gene was expressed as ‘normal’ in other Anolis and more distantly related lizards.
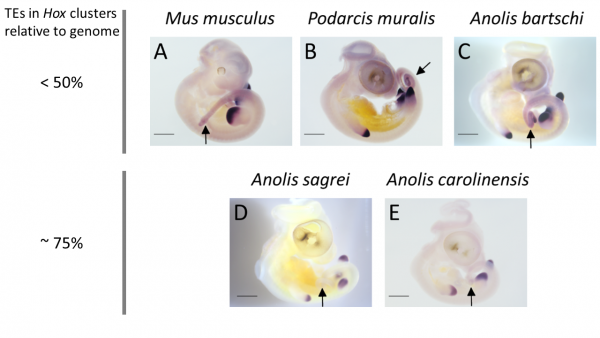
Expression patterns of the posterior Hox gene HoxD13 are showing variation between species with low and high TE content in their Hox cluster: while expression in limb buds is conserved, expression in tail tissue (black arrows) is missing in species with high TE content in their Hox clusters (A. sagrei and A. carolinensis).
Nathalie’s study reveals that, despite being THE textbook example of our conserved developmental toolkit, Hox genes can be tinkered with. What is more, the TE invasion of Hox clusters appear to be intimately linked to diversification. Now that Anolis lizards have shown us that it can happen, perhaps they can also show us why it happens and how.
The original version of this blog post was published on the Evolution Letters Editors’ Blog https://evolutionletters.wordpress.com/2019/08/06/the-role-of-mobile-genetic-elements-in-evolution-and-development/
Hot off the press!
 Our book Evolutionary Causation has arrived! It not only looks great, but it also features some really original and exciting ideas by fantastic people. Thanks to all the authors and to the KLI and the MIT Press for making it happen.
Our book Evolutionary Causation has arrived! It not only looks great, but it also features some really original and exciting ideas by fantastic people. Thanks to all the authors and to the KLI and the MIT Press for making it happen.
Interested in the content? Here is a list of titles and authors:
1 Evolutionary Causation
Tobias Uller and Kevin N. Laland
2 Causality and the Role of Philosophy of Science
Massimo Pigliucci
3 Understanding Bias in the Introduction of Variation as an Evolutionary Cause
Arlin Stoltzfus
4 The Shape of Things to Come: Evo Devo Perspectives on Causes and Consequences in Evolution
Armin P. Moczek
5 Incorporating the Environmentally Sensitive Phenotype into Evolutionary Thinking
David I. Dayan, Melissa A. Graham, John A. Baker, and Susan A. Foster
6 Genotype-Environment Interaction and the Unscripted Reaction Norm
Sonia E. Sultan
7 Understanding Niche Construction as an Evolutionary Process
Kevin N. Laland, John Odling-Smee, and Marcus W. Feldman
8 Biological Dynamics and Evolutionary Causation
Renée A. Duckworth
9 The Causes of a Major Transition: How Social Insects Traverse Darwinian Space
Heikki Helanterä and Tobias Uller
10 Are Developmental Plasticity, Niche Construction, and Extended Inheritance Necessary for Evolution by Natural Selection? The Role of Active Phenotypes in the Minimal Criteria for Darwinian Individuality
Richard A. Watson and Christoph Thies
11 The Paradox of Population Thinking: First-Order Causes and Higher-Order Effects
Denis M. Walsh
12 Ontology, Causality, and Methodology of Evolutionary Research Programs
Jun Otsuka
13 A Darwinian Dream: On Time, Levels, and Processes in Evolution
Arnaud Pocheville
14 Decoupling, Commingling, and the Evolutionary Significance of Experiential Niche Construction
Lynn Chiu
15 Biological Information in Developmental and Evolutionary Systems
Karola Stotz
Plasticity can take the lead in adaptive evolution
Plasticity-led evolution would be likely if the trait combinations induced by novel environments harbour more than their fair share of genetic variation. Our new paper in PNAS shows that this is in fact quite common. For a nice overview, see this post by Luis-Miguel Chevin on F1000.
Dan Noble and Reinder Radersma collected data from studies that quantified traits and their additive genetic covariance in animals and plants exposed to novel environments. On the whole, the multivariate phenotypic change caused by environmental change were well aligned with the maximum genetic variation – and better than expected by chance.
There are several ways to interpret these results. Perhaps the most interesting from the perspective of evolvability is that developmental systems respond to environmental novelty as they do to genetic mutation. Such developmental bias means that populations that evolve plasticity will tend to continue to evolve in some ways rather than others. The present paper is the first in a series of empirical and theoretical papers that address this problem as part of our group’s involvement in an international research effort. So please keep an eye out for more.
It is time to say goodbye…
As the EES grant comes to a close, we are sad to see some of our friends move on, but excited about the opportunities they have created for themselves.
Reinder is now a researcher in statistical genetics at Wageningen University & Research Institute, which is THE place to be for agriculture, environmental and food science. His work on maternal effects, plasticity and evolvability will undoubtedly prove useful also to domesticated plants and animals.
Alfredo also leaves us, in this case for a postdoc in Copenhagen. After a couple of years with theory, he is now ready to get his hands dirty with data again. The aim is to reveal the molecular complexities of maternal-fetal communication, a perfect opportunity to transfer skills between evolutionary and developmental biology!
Although  it is sad for all of us to see them leave, it is particularly sad for Tobias – they have known each other since the Oxford years, when Reinder did a postdoc with Ben Sheldon and Alfredo his MSc with Tobias. They have been fantastic friends and colleagues – always positive, helpful, and engaged. Hats off for everything you have taught us over the years.
it is sad for all of us to see them leave, it is particularly sad for Tobias – they have known each other since the Oxford years, when Reinder did a postdoc with Ben Sheldon and Alfredo his MSc with Tobias. They have been fantastic friends and colleagues – always positive, helpful, and engaged. Hats off for everything you have taught us over the years.
See you soon!!
Summer cottage getaway
All visitors to Sweden should experience that summer cottage feeling! Accordingly, the group packed their thermal underwear, swim trunks, shorts and rain coats and headed to Tobias’ family’s cottage in Värmland. All outfits came to good use since we had typical Swedish summer weather – blue sky followed by rain, and then glorious weather when we had to leave. Just like every year.
 View from the veranda on day 1…. and day 2…
View from the veranda on day 1…. and day 2…
Germs, lizards and field work cut our numbers this time. But the rest of us had a great time – relaxing, birding, fishing, swimming, and beaver watching, enjoying local art exhibitions, cultural history, snaps, and grillkvällar… or, to quote Yang: “we managed to do and see everything we were promised – and in addition we watched the Champion’s League final!”
 What can we catch here – pike? perch? pikeperch?… hang on, what did you just say!?
What can we catch here – pike? perch? pikeperch?… hang on, what did you just say!?
 Yang and Roman are off for an adventure! They did come back eventually.
Yang and Roman are off for an adventure! They did come back eventually.

A beaver with unfinished business, perhaps the one that swam past this morning?

Summer lunch with traditional food, snaps and songs. The students are getting younger every year…
Geoff While visiting
We are very happy to have Geoff While from the University of Tasmania visiting us for three months. Geoff is a fantastic friend and collaborator whose scientific drive, passion for biology, and lizard catching skills are nearly impossible to beat. In addition to the usual wall lizard field season, we have a few old projects to finish off, some that are in full swing, and perhaps one or two new ones to launch. Not to mention all the other things you could get done over a beer or two. Welcome Geoff!
 Cheers!
Cheers!
Ullergroup at Evolution Evolving
The Evolution Evolving at Cambridge was packed with three days of exciting talks – from culture in whales to control theory and the philosophy of explanation. The crew from Lund felt like lizards in the sun – pretty comfy and happy! So much in fact that Alfredo won the prize for best talk, and Illiam the prize for best poster. Congratulations to both!
We are very grateful to our fellow organisers and all the participants for making this such a great event. A full conference report will be posted in due time at the EES webpage, together with recordings of plenaries and key notes. They were brilliant! Until then, you can see what you missed at #evoevolving and #EES_Update
Alfredo on “How does evolution work with strong attractors?”
How adaptive plasticity evolves when selected against
Yes, that is the surprise title of Alfredo’s new paper in PLoS Computation Biology! When we discover examples of adaptive plasticity we usually think that it evolved because selection favoured it. But can adaptive plasticity evolve when selection favours non-plastic individuals?
Think of a population of insects that hatches, reproduces and dies twice every year: once in spring and once in autumn. Spring and autumn conditions may be very different, but plasticity could allow the insects to cope with both seasons. Yet, insects born in spring never experience autumn, and vice versa, so natural selection cannot directly favour plasticity. In fact, plastic individuals may do worse than individuals that are not plastic, which means plasticity could consistently be selected against.
This problem is similar to tuning the learning rate in Machine Learning. To teach a machine how to solve a problem, we give it a set of example questions we already know the answer to. Each time the machine gives us the wrong answer, we change its parameters to produce a new answer that is closer to the right one. If the examples share a consistent logic, the machine will eventually find this logic. But if we change the model’s parameters too much with every example shown, the machine will just repeat the solution for the last example, which prevents it from finding the underlying logic that connects the examples.
Just like in machine learning, organisms receive a set of questions in the form of environmental cues and need to provide the right answer in terms of a fit phenotype. Adaptive plasticity is the solution that produces the right phenotype for every environment. Organisms that see only one environment per lifetime could easily “forget” that plasticity is adaptive and instead only produce the right phenotype for their last environment. The offspring of insects that live in spring would be better matched to spring, even though they will have to deal with autumn conditions.
So when does learning theory predict that a machine will learn the logic of plasticity rather than the last solution? And do those predictions hold for a simple representation of evolution by natural selection? To find out, have a look at the paper!
Solved: the genetics of colour polymorphism in wall lizards
 A new paper in PNAS reveals that the orange and yellow ventral colour morphs in wall lizards is caused by regulatory changes in pterin and carotenoid genes. Interestingly, it seems like the alleles are old and occasionally shared between species. Miguel Carneiro and his team at CIBIO and Leif Andersson at Uppsala led the work, and we helped with the genome, samples, and other bits and pieces. The chromosome-scale genome assembly is of high quality and should become a useful resource – so just get in touch if you want to know if the wallies are right for you too!
A new paper in PNAS reveals that the orange and yellow ventral colour morphs in wall lizards is caused by regulatory changes in pterin and carotenoid genes. Interestingly, it seems like the alleles are old and occasionally shared between species. Miguel Carneiro and his team at CIBIO and Leif Andersson at Uppsala led the work, and we helped with the genome, samples, and other bits and pieces. The chromosome-scale genome assembly is of high quality and should become a useful resource – so just get in touch if you want to know if the wallies are right for you too!
A new take on the epigenetic signatures of prenatal stress
The conditions encountered in the womb can have life-long impact on health. It is usually assumed that this is because embryos respond to adverse conditions by programming their gene expression. In collaboration with Bas Heijmans and others, we propose that these effects also can be caused by selection on stochastic epigenetic variation. The paper is published in Cell Reports and there is also a press release.
The concept of fetal programming is based on the idea that embryos modify their physiology in response to the uterine environment. This may be good if conditions stay as predicted. But it may have negative health effects later in life if the conditions change. The concept of programming has been backed up by associations between adverse prenatal conditions and patterns of DNA methylation – an epigenetic mark that regulates gene expression.
Our simple insight is that the uterine environment does not need to induce changes in DNA methylation for this association between adverse prenatal conditions and DNA methylation to arise. In the early embryo, stochastic differences in gene expression can become stabilized by DNA methylation, resulting in embryos with different epigenomic profiles. Not programmed by the environment, these random differences in gene expression (and hence DNA methylation) may nevertheless provide some embryos with a survival advantage when conditions are harsh. In other words, adverse maternal conditions may impose selection on random variation in DNA methylation.
 Human embryo at 8-cell stage. (C) Yorgos Nikas/Science Photo Library
Human embryo at 8-cell stage. (C) Yorgos Nikas/Science Photo Library
We used a mechanistic simulation model to illustrate how selection reduce DNA methylation variance at loci that influence implantation success. The prediction fits very well with empirical data from offspring conceived during the Dutch Hunger Winter, a famine at the end of World War II. This makes selection on stochastic epigenetic variation a reasonable explanation for the epigenetic signatures of prenatal exposure to adverse conditions.
This new hypothesis is not only of academic interest. Fetal programming implies that the behaviour or physiology of the mother causes the offspring phenotype to change. In contrast, a selective process does not bring new phenotypes into being, it changes the distribution of already existing variants. This difference may influence which preventive strategies and treatments that are most likely to be effective. That different mechanisms can be responsible for the same pattern is also relevant to society. As Sarah Richardson has pointed out, careless discussion of epigenetic research on how early life affects health across generations could be harmful.
Developmental Bias and Evolution at Santa Fe
The third and final workshop funded by our EES grant took on the role of developmental bias in evolution. Like the meeting in February, Santa Fe greeted us with snow, jetlag, and huevos rancheros for breakfast.
The workshop showcased the extraordinary breadth of approaches used to understand if, and how, developmental processes direct evolution. Ecologists, developmental biologists, and palaeontologists explained what data are out there and what they mean. Theoretical biologists and computer scientists convincingly showed that the role of development not only can be formulated in precise terms, but that there are predictions ready to be tested. Judging from the work presented, we can look forward to some very interesting studies doing just that in the near future.
As there should be, there was both unity and spirited disagreement – with views ranging from development as constraint to development as the main driver of evolution. History and philosophy of biology again proved useful to understand this diversity. While debate over the role of development in evolution is as old as evolutionary science, contemporary research really does seem to bring new insights – conceptually, theoretically and empirically.
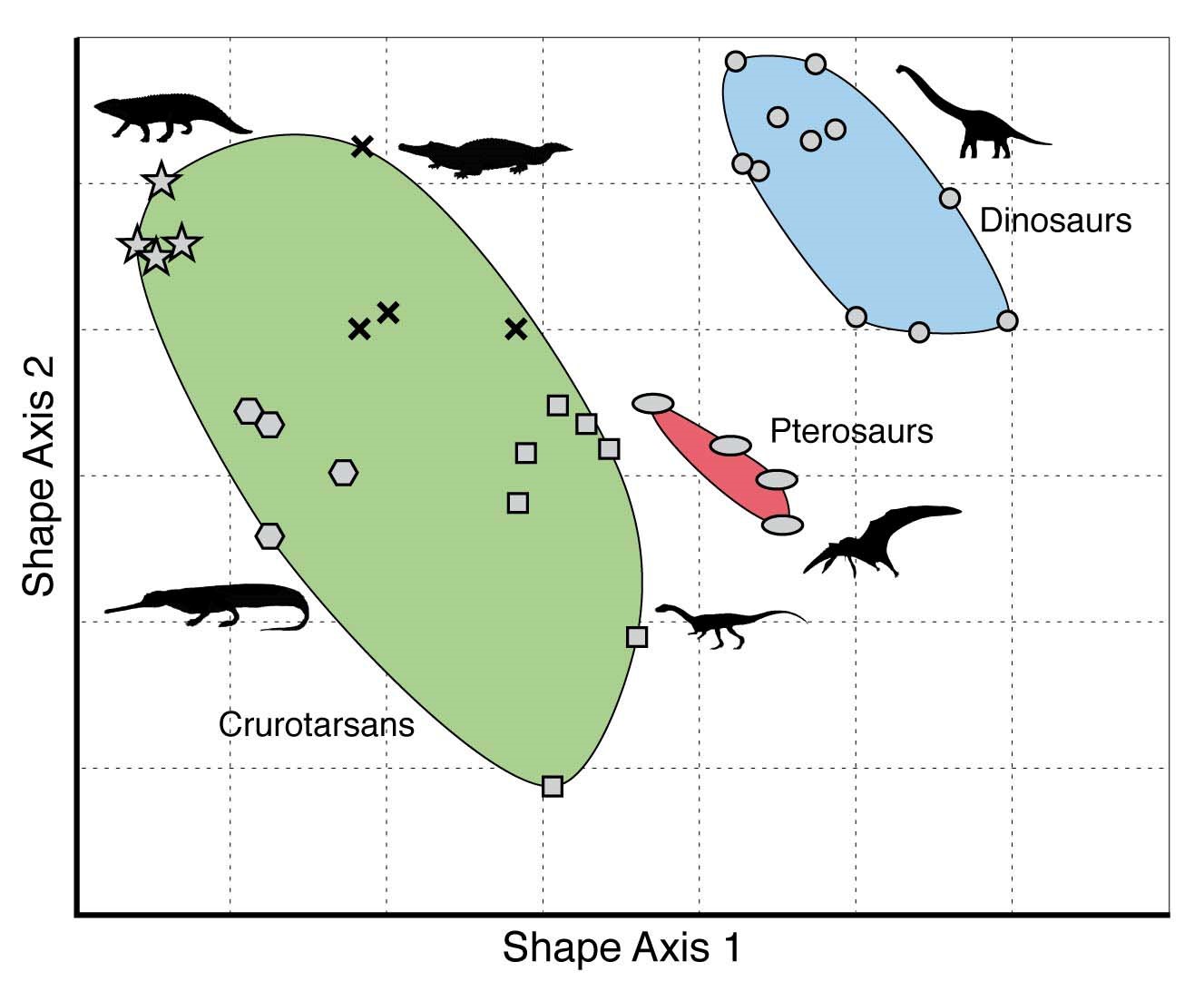
One way to think of developmental bias is through the use of morphospaces. The morphospace of several groups of reptiles (including dinosaurs) that lived around 230-200 million year ago, using two (undefined) shape characters. Reproduced from the Palaeobiology Research Group of the University of Bristol, UK
Origin-Of-Life researcher Wim Hordijk kindly summarized his views of the meeting on this blog. The Lund crew – Tobias, Alfredo, Nathalie, Reinder, and Illiam – are very grateful to the SFI for hosting, and the organisers for giving us a lot of things to think of. Next up – the Evolution Evolving meeting at Cambridge. See you there!
Another bad day for anticipatory maternal effects
A new study on water fleas, headed by Reinder and Alex, suggests that a classic example of adaptive maternal effects is not as adaptive as we might have thought.
Some years ago, Tobias, Sinead and Shinichi did a meta-analysis that seemed to undermine the idea that maternal effects are designed to transfer information about the local environment. Reviewers and editors did not really want to hear that, and the paper proved somewhat difficult to publish (but has been well received and cited). But one ‘anticipatory maternal effects’ appeared well supported: water fleas exposed to toxic cyanobacteria produced offspring that were more tolerant to the toxin.
So, to understand how this maternal effect evolves, Reinder and Alex challenged water flea mothers and offspring with cyanobacteria that produce the toxin microcystin. But rather than just expose the animals throughout their lives, they also exposed mothers at different times during her life. The rationale is that maternal effects that have been selected to transmit information are expected to behave like signals – triggered and delivered by a system well designed to pass on information with limited cost to the receiver.
It turned out that offspring indeed were somewhat better able to handle the toxin when their mothers had been exposed late in their lives. In other words, tolerance or resistance can be transmitted to the next generation through non-genetic means. But there was not much to suggest that the mechanism has been selected to transmit information about the presence of cyanobacteria. Instead, our experimental evidence – along with a meta-analysis of Daphnia research – fits better with a model of passive transfer of tolerance rather than an anticipatory maternal effect. Again, reviewers and editors were not very happy to hear this, and this paper also proved somewhat difficult to publish.
We should not be too surprised of these results, however. Not all plastic responses that allow organisms to cope with stress are properly seen as adaptations to cope with the stressor.
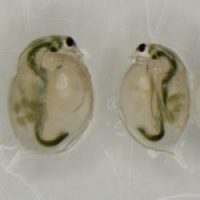
Genetically identical sisters, raised in two different environments. Daphnia raised in the microcystin treatment (on the right) displays smaller body size and carries less eggs than her sister from the control treatment
We can think of these maternal effects as – in Mary-Jane West-Eberhard terms – examples of phenotypic accommodation that have not yet been followed by genetic accommodation.
But while our results question if there really has been selection for maternal transfer of information about microcystin exposure, the study is still not quite conclusive. Two kinds of follow-up studies would be particularly informative.

Endless replications
The first is to contrast responses of populations of water fleas with different evolutionary histories of exposure. This kind of comparison can effectively reveal if plastic responses in naïve animals become fine-tuned over evolutionary time (see a nice example from house finches here).
The second is to reveal the mechanism of the maternal effect. This would not only tell us ‘how it works’, but – keeping the comparison with signals in mind – whether or not the mechanism has properties that we expect of systems designed to transmit information.
We have our ideas about what is going on, and Alex has just completed a set of experiments that can tell us more. In the meantime, we hope that this paper will inspire more studies to look at maternal effects as a window into the evolutionary transition from stress-induced responses to local adaptation. To get started, please see here and here.
Lund workshop on the evolutionary origins of individuality
The evolutionary process is itself evolving. Perhaps the best example is the origin of new reproductive organizations that are capable of evolving by natural selection. Single celled organisms evolved into multicellular organisms, some of which – leaf cutter ants, for example – appear to have evolved into collectives that deserve the label superorganism.
Last week our group hosted a small workshop to bring together researchers that use different approaches to tackle how and why such transitions in individuality happens. The aim was to share insights from projects within our Templeton-funded grant, and to spread ideas to local Lund researchers.
The presentations generated a lot of great discussions, which continued over dinner and at the pub. Richard Watson showed how learning theory bring clarity to the conditions that are necessary for transitions in individuality to happen. Andy Gardner explained how focusing on lower-level evolutionary individuals – genes – can help to understand how conflicts between cells within bodies arise, evolve and become resolved. Charlie Cornwallis and Maria Coelho-Svensson discussed how one can put theory to the test using green algae that come together in groups. Social insects provide another interesting example, and Heikki Helanterä showed how they can shed light on the transition from solitary to collective life cycles.
Evolutionary transitions in individuality is fundamentally about explaining how a developmental organization evolves from social or ecological interactions between the lower-level individuals. This is difficult to address without paying attention to mechanism. Miguel Brun-Usan showed how mechanistic models of cell behaviour provide a window into the conditions that promote transitions in individuality. Some of the order comes from self-organization, a topic theoretically explored by Edith Invernizzi in the context of nest building in social insects. Finally, Jonathan Birch discussed another evolving relationship between the organism and its surroundings – the evolutionary route towards organisms that are capable of conscious thought.
Thanks to all the speakers and the many Lund researchers and students that attended the workshop. It was great fun and we cannot wait to see more from all of these projects.
Developmental Bias and Evolution
A recurrent theme in evolutionary biology is to contrast natural selection and developmental constraint – two forces pitted against each other as competing explanations for organismal form. A recent Perspective in the journal Genetics explains why this juxtaposition is deeply misleading. There is also a blog about the paper here.
Our starting point is that characters often evolve through changes in how genes, cells, and tissues regulate each other. Once we study the evolution of such regulatory interactions, it turns out that they do not only limit evolution, but also may facilitate the capacity to adapt and diversify. The paper gives an introduction to recent theoretical and empirical research, and explains how this work is now beginning to reveal how evolution of the evolutionary process itself contributes to diversification and adaptation.
Three new papers on thermal biology of reptile embryos
Most reptiles lay eggs in sand or soil, under logs or in rock crevices. These are places where the temperature often fluctuates, sometimes becoming dangerously high or low. The pervasive effects of temperature on biological systems begs the question how embryos respond in the short term, and how populations adapt in the long term. This question is now thoroughly explored in a theme issue of the Journal of Experimental Zoology, with contributions from our group.
In a study on wall lizards led by Nathalie Feiner, we reveal a pervasive effect of temperature on gene expression in early embryos. The strongest responses were found for genes involved in transcriptional and translational regulation and chromatin remodelling, suggesting possible epigenetic mechanisms underlying thermal acclimation. In another paper, ploughing through 50 years of experimental studies, Dan Noble, Geoff While, and colleagues summarize research on thermal plasticity in reptile embryos, and demonstrate how future progress can be made through meta-analytic and comparative work. The data are accessible to everyone through a paper in the journal Scientific Data, and can be downloaded through this online data base. More papers in this special issue are available through the Journal of Experimental Zoology online early.